Radiation Physics and Biology
Home > Radiation Protection and Quality Assurance > Radiation Physics and Biology
Can we please get your advice on this one question?
Radiation Physics
Atoms and Nucleus
- Matter is made up of atoms.
- Atoms in turn are made up of electrons neutrons and protons.
- Neutrons and protons are found in the nucleus and electrons in a cloud of orbits around the nucleus.
- Protons have a positive electric charge and neutrons have no electric charge.
- An electron has a negative electric charge of 1.6×10-9 coulombs which is equal in magnitude to the charge of a proton.
- Atoms differ from each other in the constitution of the nucleus and in the number and arrangement of the electrons.
- Atoms of an element with the same number of protons (atomic number) but different number of neutrons (atomic mass) are referred to as isotopes of an element
- They
can be stable or unstable. Unstable isotopes can become stable by ejecting
extra neutrons in a process called nuclear disintegration or radioactivity.
Radioactivity
- Radioactivity is specifically the rate at which decays occur.
- It is accompanied by emission of ionizing radiation or particles or both.
- The amount of radioactivity present depends on the number of radioactive atoms and their corresponding half-life.
- Half-life of an isotope is the time required for the half of the number of atoms in the sample to decay.
- The unit of radioactivity is either Becquerel (Bq) or Curie (Ci), where; 1 Becquerel is equal to 1 disintegration per second, and 1 Curie is equal to 37 billion disintegrations per second.
- The number of disintegration is directly proportional to the number of radioactive atoms present.
Types of Ionizing Radiations
There are three primary types of ionizing radiations; alpha particles (α), beta particles (β), and photons.
1. Alpha particles
- They are emitted with a positive charge of 2.
- They lose energy rapidly and stop in a very short distance, no more than 5 centimeters in air and about 30-50 micrometers in water and or tissue.
- Therefore external alpha emitters therefore are generally not considered to pose a health hazard.
2. Beta particles
- They are emitted with either 1 negative charge (electron) or 1 positive charge (positron).
- They lose energy slowly when passing through materials.
- High-energy beta particle can travel several centimeters through water and tissue while lower-energy beta particles travel some fraction of that distance.
- Therefore external emission of low-energy beta particles cannot cause any health hazard.
3. Photons
They are either x rays or gamma rays (γ).
They have no charge and are thus neutral.
They have high penetrating power.
High-energy x rays and gamma rays travel many meters in air and through many centimeters of concrete, iron, and tissue.
Thus, external gamma rays can penetrate and
deposit energy throughout the body, whereas internally emitted gamma rays can
deposit energy in the tissue of residence or neighboring tissues.
- Sources of Radiation
- Basic Properties of Radiation
- Interactions with Matter
- Biological Effects of Radiation
- Measurement of Radiation
Fifteen; 15 questions out of the 35 questions in the Radiation Protection and Quality Assurance content area come from the Radiation Physics and Biology sub-topic.
Let's start with the basic definition of radiation. Radiation is the process in which energy is emitted as particles or waves. Common examples of radiation are the music we listen to as well as the electromagnetic properties used to run our mobile phones. In the field of radiation therapy, we consider how energy emitted; which is, radiation can be used in the treatment of cancer.
Although we may not think about it, radiation is and has always been all around us. Sources of radiation come from natural as well as man-made sources.
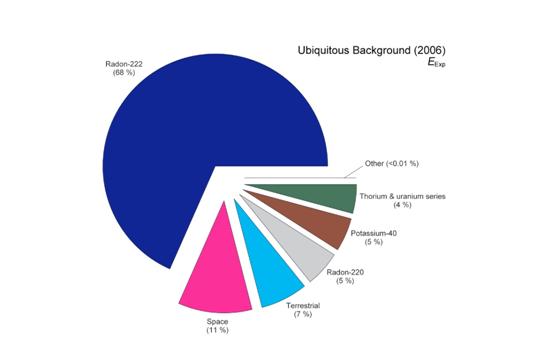
There are three principal areas which make up natural sources of radiation. The first area of these natural sources is radon. You cannot see or feel radon. Radon gas comes from the ground and is the product of the decay of radioactive material. Radon is a major factor in the cause of lung cancer.
http://www.cancer.gov/cancertopics/factsheet/Risk/radon
A second area of natural sources of radiation is cosmic or space radiation. One every day example of the cosmic radiation we receive is sunshine. Indeed, we are constantly bombarded by radiation from space, similar to a steady drizzle of rain. Charged particles from the sun and stars interact with the earth’s atmosphere and magnetic field to produce a shower of radiation that we are all exposed.
A third area of natural sources of radiation is called terrestrial radiation. When we use the word terrestrial we mean natural sources of radiation “other than radon”. These sources may be internal sources; which means, they are actually present in our bodies. Radiation is present in our bodies coming from the foods we eat as well as the world we live in. For example, the bananas we eat have potassium and some of this potassium is radioactive. Cigarette smoke is also a source of internal radiation.
http://www.epa.gov/radiation/sources/tobacco.html
In addition to internal sources, radiation is everywhere in our environment. Please see the image below which is a pie chart of natural radiation sources.
http://www.ncrponline.org/images/160_pie_charts/Fig3-19.pdf
Radiation also comes from man-made sources. About half of the man-made sources come from medical radiation exposure. Some examples of medical radiation exposure include: CT scan, x-rays, as well as nuclear medicine studies. In the United States, radiation emitting products are controlled by the US Food and Drug Administration. Of course, in our field of radiation therapy we are also concerned with occupational exposure.
http://www.fda.gov/Radiation-EmittingProducts/default.htm
There are many other sources of man-made radiation. These other sources include smoke detectors, luggage security screening, radiation-treated materials like food as well as the mail, and industrial instrumentation. Learn more about other sources of man-made radiation at the US Environmental Protection Agency educational website called RadTown.
http://www.epa.gov/radtown/topics.html
Basic properties of radiation are different for different type of radiation. Radiation is energy transmitted through space in the form of electromagnetic waves or energetic particles. Electromagnetic radiation, like light or radio waves, has no mass or charge. Types of radiation and its properties are clearly shown in the Electromagnetic Spectrum diagram.
The radiation is classified two broad categories,
- Ionizing Radiation (Alpha, Beta, and Gamma)
- Non-Ionizing Radiation (Radio waves, Visible light, Microwaves etc.)
Basic properties of Ionizing Radiation:
- Alpha Particle:
- Alpha particles are positively charged particle and have mass
- The range of the alpha particles is less than the beta and gamma
particle
- Alpha particles are heavily charged particle because of this it will undergo dense ionization when interact with matter.
- The penetrating power of alpha particles is less than the beta and gamma particles. It can stop by thick paper itself.
- The specific ionization of alpha particles are higher than the other particles or rays.
- It will affect the photographic plate
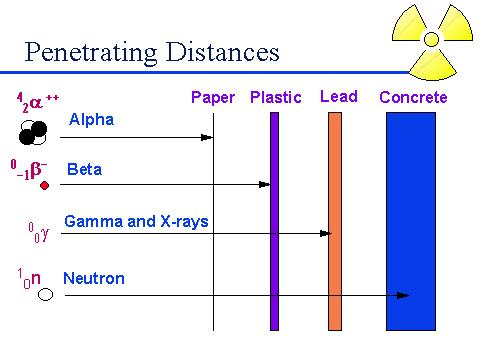
The
above diagram shows the penetrating power of alpha, beta, gamma, and neutron.
- Beta
Particles:
- Beta particles are otherwise call it as electrons, the mass of the beta particles is 1800 times less than the alpha particles.
- The range of the beta particles is higher than the alpha but, lesser than the gamma rays.
- The penetrating power of the beta particles is slightly higher than the alpha but, lesser than the gamma rays. It requires few mm of Al to absorb it.
- Beta particles also affect the photographic plate
- Specific ionization less than the alpha
particles.
- Gamma
Rays:
- Gamma photons are not having the mass.
- Gamma photons can act as both particle as well as wave characteristic.
- It will not deflected by electric and magnetic field
- The range of gamma rays is higher than the alpha and beta particles an less than the neutron
- Gamma rays are sparsely ionizing radiation.
- Specific ionisation of gamma rays are very less when compared to aloha.
- Gamma rays are absorbed by the a few cm thickness of lead.
- It will affect the photoelectric effect
Exponential Attenuation
The concept of exponential attenuation is relevant primarily to uncharged ionizing radiation (i.e., photons and neutrons), which lose their energy in relatively few large interactions, rather than charged particles which typically undergo many small collisions, losing their kinetic energy gradually. An individual uncharged particle has a significant probability of passing straight through a thick layer of matter without losing any energy, while a charged particle must always lose some or all of its energy.
An uncharged particle has no limiting “range” through matter, beyond which it cannot go; charged particles all encounter such a range limit as they run out of kinetic energy. For comparable energies, uncharged particles penetrate much farther through matter, on the average, than charged particles, although this difference gradually decreases at energies above 1 MeV. Consider a monoenergetic parallel beam consisting of a very large number N0 of uncharged particles incident perpendicularly on a flat plate of material of thickness L.
Simple Exponential Attenuation:
Assume for this ideal case that each particle either is completely absorbed in a single interaction, producing no secondary radiation, or passes straight through the entire plate unchanged in energy or direction
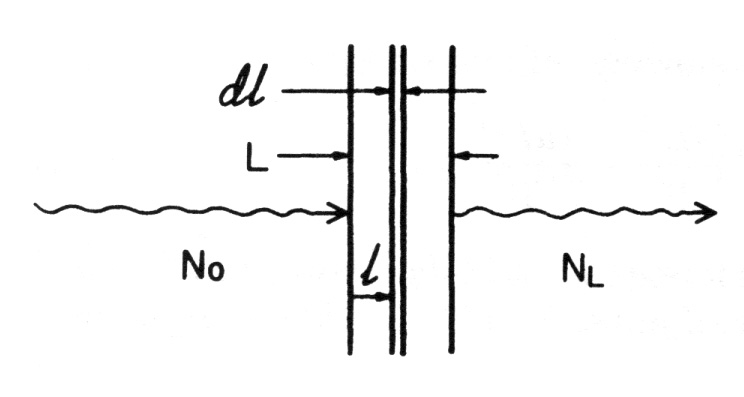
- Let (µ.1) be the probability that an individual particle interacts in a unit thickness of material traversed
- The probability that it will interact in an infinitesimal thickness dl is mdl
- If N particles are incident upon dl, the change dN in the number N due to absorption is given by
dN = -µdl
where µ is typically given in units of cm-1 or m-1, and dl is correspondingly in cm or m
- The fractional change in N due to absorption of particles in dl is just
dN/N = -µdl
- Integrating over the depth l from 0 to L, and corresponding particle populations from N0 to NL, give
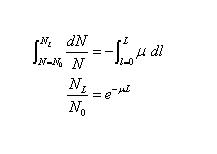
- This is the law of exponential attenuation, which applies either for the ideal case described above (simple absorption, no scattering or secondary radiation), or where scattered and secondary particles may be produced but are not counted in NL
- The quantity µ is called the linear attenuation coefficient, or simply the attenuation coefficient
- When it is divided by the density r of the attenuating medium, the mass attenuation coefficient m/r (cm2/g or m2/kg) is obtained
- µ is sometimes referred to as the “narrow-beam attenuation coefficient”
Inverse Square Law
Inverse Square Law is defined as intensity is proportional to the square of the distance.
INTENSITY ∞ 1/ (DISTANCE)2
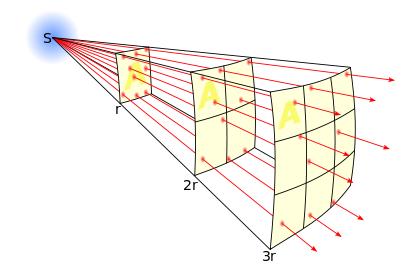
The lines represent the flux emanating
from the source. The total number of flux lines depends
on the strength of the source and is constant with increasing distance. A
greater density of flux lines (lines per unit area) means a stronger field. The
density of flux lines is inversely proportional to the square of the distance
from the source because the surface area of a sphere increases with the square
of the radius. Thus the strength of the field is inversely proportional to the
square of the distance from the source.
Applications of Inverse Square Law:
- Radiation Protection
- Nuclear Medicine
- Radiation Protection:
Inverse square plays a very important role in reducing of radiation hazards in the field of radiotherapy. Basically to implement the radiation protection in the radiotherapy department three things needs to be done.
- Time
- Distance and
- Shielding
Out of these we going to see the distance part in brief now.
In Radiation Protection point if you spend more distance from any source of radiation the concern person will be exposed less than the near one. As discussed earlier above the distance increases the intensity decreases and vice versa. Because of this only distance is consider as the most priority one however, when we establishing the radiation facility it is not possible to leave required amount of space. To resolve this problem only third one came into picture that’s Shielding.
- Nuclear Medicine:
Inverse square law is having a lot of advantage in nuclear medicine also. Because in nuclear medicine the technologist or nuclear medicine physician are handling the source by hand in that time they are prone to expose themselves. So, that inverse square law is applied their also to reduce the exposure from the radioactive source. Tongs, Elbow-Tap, lead container etc. are used to minimise the exposure.
X-ray Beam Quality
All X-ray units do not always produce the same quality of X rays for a given voltage. This may be due to incorrect calibration, age of apparatus, waveform and other causes. Unless the beam quality is known, dose measurements and tests on radiographic recording systems may be invalid. It has been shown (Ardran and Crooks, 1963) that a difference of 10 kV can affect the patient integral dose by 20-40 per cent. Diagnostic X-ray beam quality is usually expressed by means of the kV or kVp and the filtration or, alternatively, the half-value thickness (HVT) in millimetres of aluminium. However, HVT alone is not a good indicator of beam quality for this purpose: a simple experiment will show that it is possible, with different kVp's and filtrations, to produce X-ray beams with the same HVT but which give radiographs of quite different quality. This point has been stressed by Holm and Moseley (1964) and by other workers. HVT is, of course, valuable when the kVp is known, particularly if similar wave forms are being considered.
The Factors that will affect the X-ray beam quality are KVp.
- X-ray Beam Quality (KVp)
The quality of the x-ray beam is controlled by the amount of voltage. Voltage provides contrast to the film.
The desired contrast appears as various shades of gray, black, and white in the x ray negative (radiograph).
- Increased voltage provides less contrast (or more shades of gray). However, the beam has more penetrating power. Decreased voltage, on the other hand, provides more contrast (fewer shades of gray and more black and white shades). However, there is less penetrating power in the low voltage exposure. The technique most commonly used to expose periapical and bite-wing X-raysis a 75 kilovolt peak and 15 milliamperes.
Beam Quality Defining Factor (HVL):
Half Value Layer (HVL) is used to define the X-ray beam quality. The HVL of an x-ray beam is the amount or thickness of absorbing material or filtration that must be placed in the beam to reduce the transmission of the beam by one half. The material predominantly used to determine the HVL of diagnostic xray equipment is aluminum, and hence HVL is expressed in terms of mm of aluminum. Medical diagnostic x-ray machines typically have HVLs ranging from 2.3 to 5 mm Al. Total filtration and HVL are sometimes erroneously used interchangeably. They are not equivalent terms. The introduction of a known thickness of aluminum will not increase the HVL by a corresponding amount of millimeters of aluminum. For example, in the following table at 80 kVp, 1.5 mm Al total filtration results in an HVL of 1.8. An increase of 1 mm Al to a total of 2.5 mm Al changes the HVL to 2.4 mm, an increase of only 0.6 mm Al. While the total filtration doesn’t typically change, the HVL measured will vary depending on the kVp and the type of high voltage generator (i.e., single phase, three phase, high-frequency, etc.) used in the machine.
- Interactions with Matter
Electromagnetic Radiations are classified into two one is Ionizing radiation (x-ray photon, gamma photon, particle radiation etc.) and another one is Non-ionizing radiation (radio waves, microwaves etc.). Out of these two Ionizing radiations are more harmful to environment, human beings and other living creatures in the world. The attenuation of photon beam by an absorbing material caused by five major interactions such as Photo disintegration (P.D), Rayleigh scattering (R.C), Photoelectric Effect (P.E), Compton Effect (C.E), and Pair Production (P.P). The total attenuation coefficients are,
Total attenuation coefficient = P.D+P.E+C.E+P.P+R.C.
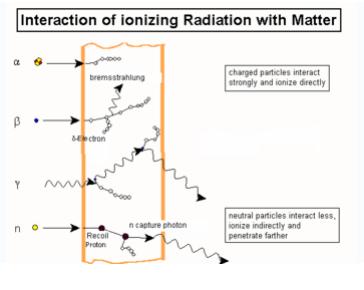
Figure shows the different types of interactions by ionizing radiations.
The modes of interactions of ionizing radiations are mentioned below;
- Photodisintegration:
Photodisintegrations is the process in which high energy photons interacts with the atomic nucleus and causes it to an excited state and back by emitting an subatomic particles such as proton, neutron.
- Rayleigh Scattering:
When a photon interacts with atom, it may or may not impart some energy to it. The photon may be deflected with no energy transfer. This process is called Rayleigh scattering and is most probable for very low-energy photons
- Photoelectric Effect:
Photoelectric effect is one type of interactions by photons in which the photon interacts with one of the orbital electron and ejects also. Photoelectric effect is predominant mode of interactions at low energies.
- Compton Effect:
In Compton Effect, photon interacts with free electron and ejects from its orbit, and photon travels in different direction part of its energy. Compton Effect is predominant in medium energies between 100KV to 10MV.
- Pair Production:
Pair production occurs when Photons energy greater than 1.024 MeV, under the influence of the electromagnetic field of a nucleus, may be converted into electron and positron. At least 1.024 MeV of photons energy are required for pair production, because the energy equivalent of the rest mass of the electron and positron is 0.51 MeV each. Pair production is not very probable, however, until the photon energy exceeds about 5 MeV.
- Electron Interactions
Beta particles can interact with electrons as well as nuclei in the medium through which they are traveling. Beta particles passing near nucleus will be deflected by the coulomb forces and losses of the beta particles kinetic energy may or may not (Rutherford scattering) occur.
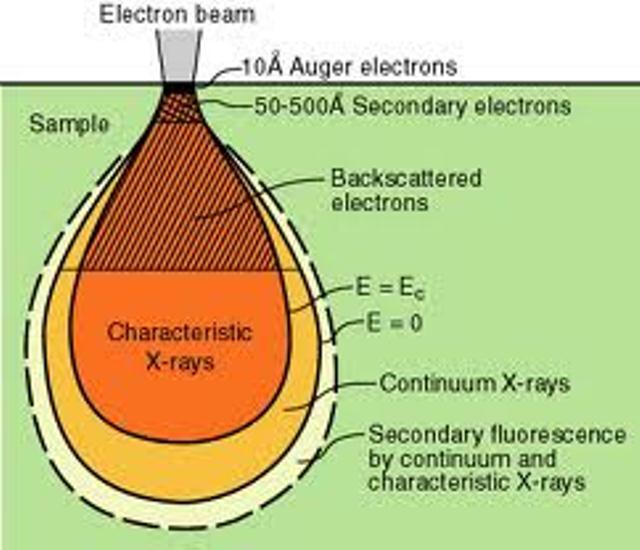
Figure shows the electron interaction with matter
The interactions of beta particles with orbital electrons are most important. Coulomb repulsion between beta particles and electrons frequently results in ionization. In the ionization process, the beta particles lose an amount of energy equal to the kinetic energy of the electron plus the energy used to free it from the atom. A beta particle may produce 50 to 150 ion pairs per centimeter of air before its kinetic energy is completely dissipated. The characteristic X-rays are emitted, when the vacant internal electron orbits are refilled with other electrons. Beta particles also cause excitation of external orbital electrons, which in turn leads to the emission of ultraviolet photons.
The ultimate fate of a beta particle depends upon its charge. A negatively charged beta particle, after its kinetic energy has been spent, either combines with a positively charged ion, or becomes a "free electron". Positrons, however, have a different fate. In spite of the fact that they dissipate their kinetic energy just like beta particles through ionization and excitation, they cannot exist at rest in the vicinity of the electrons. When a positron has been slowed sufficiently, it will be attracted to the opposite charge of an electron. When the electron and positron collide, they are both annihilated and an amount of energy equal to the sum of the particle masses is released in the form of two photons. These photons are referred to as "annihilation radiation". Both annihilation photons carry energy of 0.512 MeV, which is equivalent to the rest mass of the electron or the positron. Because of this phenomenon, 0.512 MeV photons often provide a convenient means for measurement of positron-emitting radionuclides.
Like alpha particles, betas have a characteristic average traveling distance (range) through matter that is dependent upon their initial kinetic energy. Beta particle range may be expressed as distance traveled in a certain medium. For example, beta particle with energy about 2 MeV will travel up to 9 m in air and about 10 mm in water.
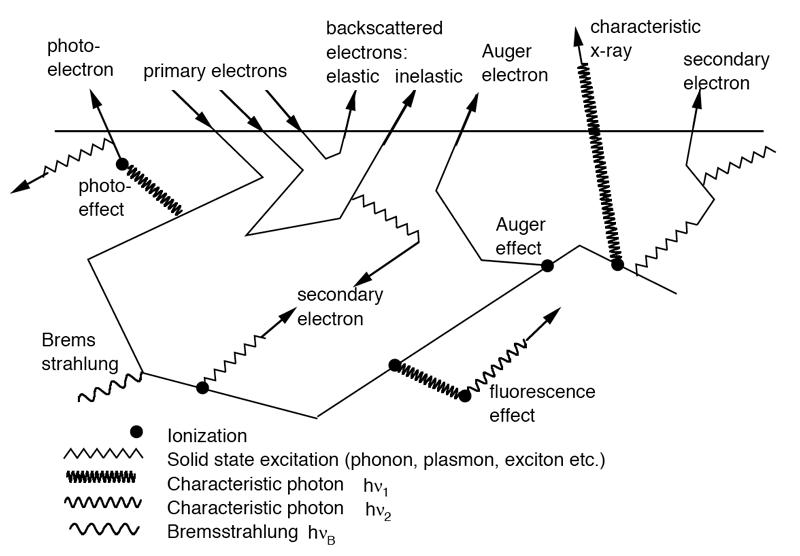
Figure shows the interactions electrons in matter
- Particle Interactions
Particle Radiation is known till now in the field of radiotherapy are Alpha Particle, Proton, Electron, and Radiotherapy. The mode of interaction in the matter is different for different particle not same as photons. Let us see one by one briefly here.
Alpha particle:
The most probable process involved in the absorption of alphas, however, are ionization and excitation of orbital electrons. Ionization occurs whenever the alpha particle is sufficiently close to electron to pull it out from orbit though coulomb attraction. Each time this occurs, the alpha loses kinetic energy and is thus slowed. The alpha also loses kinetic energy by exciting orbital electrons with interactions that are insufficient to cause ionization. As it becomes slowed, the alpha has tendency to cause ionization at an increasing rate. As the alpha nears to the end of its track, its rate of ionization peaks and within very short distance, it stops, collects two electrons and becomes helium atom.
Because alpha particles are comparatively heavy and have a charge, they react strongly with matter, producing large numbers of ions per unit length of their path. As a result, they are not very penetrating. For example, 5 MeV alpha particles will only travel about 3.6 cm in air and will not penetrate an ordinary piece of paper. For the other materials the average travel distance with respect to air is approximately inversely proportional to the respective densities of each material. 5 MeV alpha particles will only travel about 4 μm in mammal tissue.
Electron or Beta particles:
Beta particles can interact with electrons as well as nuclei in the medium through which they are traveling. Beta particles passing near nucleus will be deflected by the coulomb forces and losses of the beta particles kinetic energy may or may not (Rutherford scattering) occur.
The interactions of beta particles with orbital electrons are most important. Coulomb repulsion between beta particles and electrons frequently results in ionization. In the ionization process, the beta particles lose an amount of energy equal to the kinetic energy of the electron plus the energy used to free it from the atom. A beta particle may produce 50 to 150 ion pairs per centimeter of air before its kinetic energy is completely dissipated. The characteristic X-rays are emitted, when the vacant internal electron orbits are refilled with other electrons. Beta particles also cause excitation of external orbital electrons, which in turn leads to the emission of ultraviolet photons.
Proton Interactions:
The proton interactions with atomic electrons are generally inelastic; that is, the proton loses a small amount of energy by ionizing atoms (kicking an electron out of its orbit). These interactions generally do not result in much change to the proton direction, but many scatters do reduce the proton energy. With dense materials, the energy loss can be quite large (100 to 500 mega electron volts). The amount of energy loss can be important, depending on the energy of the beam.
Protons travel a few centimeters to tens of centimeters through matter before they undergo a significant interaction with the object either through strong or electromagnetic forces. These so-called interaction or attenuation lengths are optimum for radio graphing objects to extract precise physical characteristics such as density. In contrast, x rays have a maximum attenuation length of about 1 centimeter. For a 10-centimeter-thick slab of material, a beam of x rays is reduced by roughly one million (one in a million x rays makes it through the slab), whereas one in three protons makes it through. Because many more protons make it through than x rays, scientists say that protons are more penetrating.
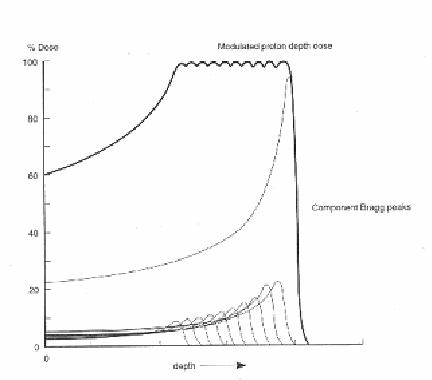
- Neutron Interaction:
The neutrons interact with atoms due to electromagnetic and nuclear forces. The neutrons affect living matter by the process of moderation. A high-energy neutron encountering biological material is apt to collide with a proton with sufficient force to dislodge the proton from the molecule, which held it. The proton (normally called the "recoil proton") may then have sufficient energy to travel some distance in the tissue causing secondary damage through ionization and excitation of molecules along its path. The most efficient absorbers of a neutron beam are the hydrogenous materials such as paraffin wax or polyethylene. Lead, which is a very good absorber for x-rays, is a poor shielding material against neutrons.
Biological effects of radiation are typically divided into two categories. The first category consists of exposure to high doses of radiation over short periods of time producing acute or short term effects. The second category represents exposure to low doses of radiation over an extended period of time producing chronic or long term effects. High doses tend to kill cells, while low doses tend to damage or change them. High doses can kill so many cells that tissues and organs are damaged. This in turn may cause a rapid whole body response often called the Acute Radiation Syndrome (ARS). High dose effects are discussed below. Low doses spread out over long periods of time don’t cause an immediate problem to anybody organ. The effects of low doses of radiation occur at the level of the cell, and the results may not be observed for many years.
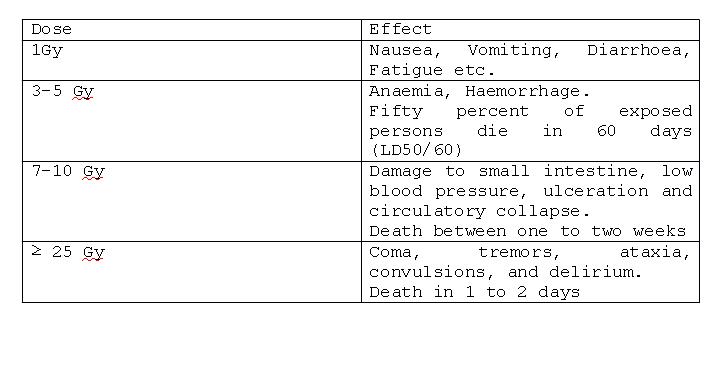
Acute Radiation Syndrome (ARS):
ARS is having three types syndrome,
- Haematopoietic Syndrome
- Gastrointestinal Syndrome
- Central Nervous System
As the dose increases above 1.5Gy these three systems manifest it depending on the dose levels. The signs and symptoms of the ARS are nausea, vomiting, fatigue and loss of appetite.
Syndrome Organs Affected Sensitivity
Hematopoietic Blood forming organs Most sensitive
Gastrointestinal Gastrointestinal system Very sensitive
Central Nervous System Brain and muscles Least sensitive
In summary, radiation can affect cells. High doses of radiation affect many cells, which can result in tissue/organ damage, which ultimately yields one of the Acute Radiation Syndromes. Even normally radio-resistant cells, such as those in the brain, cannot withstand the cell killing capability of very high radiation doses.
- Effects of Low Doses of Radiation:
There are three general categories of effects resulting from exposure to low doses of radiation. These are:
- Genetic - The effect is suffered by the offspring of the individual exposed (mutations).
- Somatic - The effect is primarily suffered by the individual exposed. Since cancer is the Primary result, it is sometimes called the Carcinogenic Effect.
- In-Utero - Some mistakenly consider this to be a genetic consequence of radiation exposure, because the effect, suffered by a developing embryo/fetus, is seen after birth. However, this is actually a special case of the somatic effect, since the embryo/foetus is the one exposed to the radiation.
Exposure to radiation is not a guarantee of harm. However, because of the liner, no-threshold model, more exposure means more risk, and there is no dose of radiation so small that it will not have some effect.
- Somatic Effects
Somatic Effects are an effects suffered by the individual exposed. Primary consequence is cancer. Somatic effect is divided into types one is Deterministic Effect and Stochastic Effect.
- Deterministic Effects:
Deterministic effects are the effects in which the severity of the effect increases once it’s reached the threshold dose for the particular organ or tissue etc. no effect will be observed below Gy of dose. Examples for deterministic effects are cataract, organ failure, and sterility etc. figure below shows the effects lucidly.
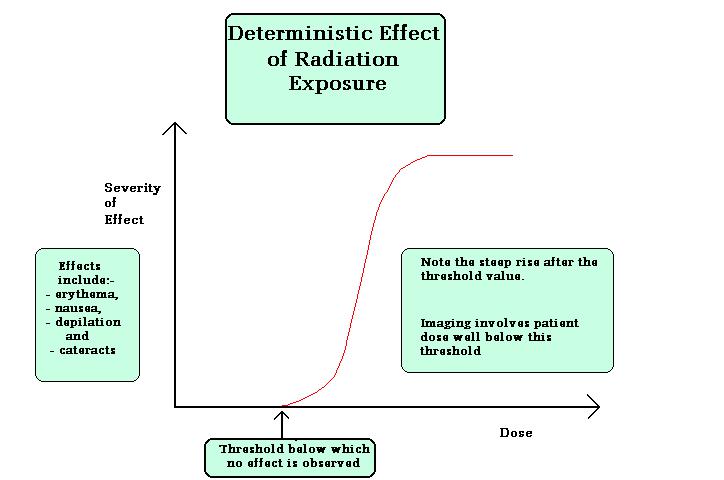
Doses required to produce deterministic effects:
Several factors may dramatically modify the effect of absorbed doses of ionizing radiation, including
- LET
- Dose and its distribution in time,
- Uniformity of irradiation,
- Age at exposure,
- Sex
- Genetic susceptibility,
- Adaptive response,
- Kind and degree of medical care, and
- Other factors.
- Stochastic Effects:
Stochastic Effects is the effect in which the severity of the effect is not dependent on any threshold dose, probability occurrences is more at low doses also. Two types of stochastic effects are induction cancer, genetic effects.
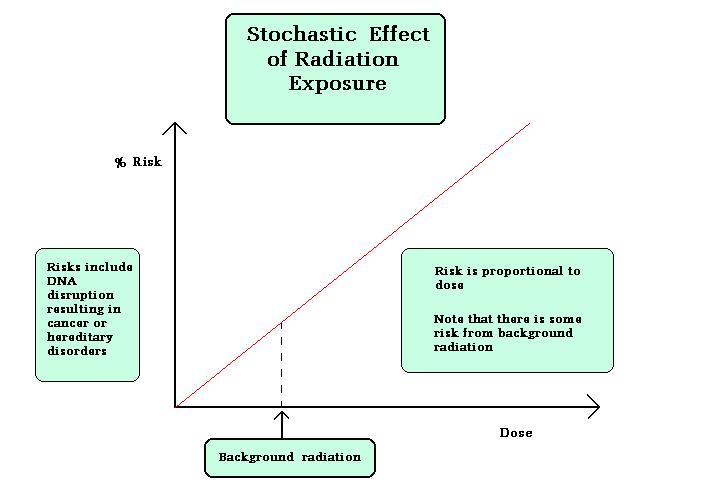
Figure explains the stochastic effect graphically.
- Cancer Induction:
Some of the population studied and their associated cancers are:
- Lung cancer - uranium miners
- Bone cancer - radium dial painters
- Thyroid cancer - therapy patients
- Breast cancer - therapy patients
- Skin cancer - radiologists
- Leukemia - bomb survivors, in-utero exposures, radiologists, therapy patients
- Genetic Effects:
Genetic effects are due to modification in germ cells (sperm or ovum). Not sure that this effect will occur immediately it may after a few generations, Study taken in atomic bomb survivors in japan reveals this effect significantly. Till now 500 genetic effects has been found in the world.
As Low As Reasonably Achievable (ALARA) principle is imposed to reduce the stochastic effects and permanently avoid the deterministic effects.
- Units of Measurements
Units of measurements are different for different medium (air, water). Till now below mentioned units are used to find the absorbed dose and also to represent the dose limits in the field of radiotherapy. They are,
- Exposure
- Absorbed Dose
- Equivalent Dose
- Effective Dose
Exposure (X):
It is defined as the quotient of dQ by dm where dQ is the absolute value of the total charge of the ions of one sign produced in air when all the electrons (negatrons and positrons) liberated by photons in air of mass dm are completely stopped in air.
X= dQ/dm
The SI unit of exposure is C/Kg and special unit is Roentgen.
- Absorbed Dose:
It is defined as the absorbed dose, or simply dose, is the quotient where dE/dm is the mean energy imparted by ionizing radiation to material of mass dm (1). The old unit of dose is rad (an acronym for radiation absorbed dose) and represents the absorption of 100 ergs of energy per gram of absorbing material.
The SI unit of absorbed dose is Gy and it is defined as ,
1 Gy = 1J/Kg
The relationship between the Gray and Rad is
1Gy = 100rad
- Equivalent Dose (H):
As we know that different types of radiation are used for the treatment of cancer, all the radiation will not produce the same biological effect. It will be different for different radiation like alpha, beta, and gamma etc. so, that when we are encounter the absorbed dose to the person should include this phenomenon also to consider.
It is defined as the product of absorbed dose and the radiation weighting factor (wr).
H = D x wr
The SI unit of Equivalent Dose is Sievert (Sv). One Sievert is equal to 100 rem
The radiation weighting factors for different types of radiation are mentioned below Radiation weighting factors
- X-rays, gamma rays, beta particles, and electrons––––––––– 1
-
Protons
(>2 MeV) and charged ions ––––––––––––––––––––––––– 2
-
Alpha
particles and other multiple-charged particles –––––– 20
- Neutrons––––––––––––-- A Continuous function of Neutron Energy
- Effective Dose (E):
Not only that the different types of radiation produce different biological effect , that biological effect is also vary according to the sensitive organ or tissue for the radiation. For that ICRP 60 introduced the tissue weighting factors for different organs and tissues are shown in the table below.
Effective dose is defined as the product of Equivalent Dose and Tissue weighting factors (wt)
E = ∑T x wT x HT
The SI unit of effective dose is same as equivalent dose.
Recommended tissue weighting factors:
Tissue Tissue weighting factor, wT Sum of wT values
Bone-marrow (red), 0.12 0.72
colon, lung,stomach,
breast, remainder tissues (a)
Gonads 0.08 0.08
Bladder, oesophagus, 0.04 0.16
liver, thyroid.
Bone surface, brain, 0.01 0.04
salivary glands, skin.
Total 1.00
Remainder tissues: Adrenals, extrathoracic (ET) region, gall bladder, heart, kidneys,lymphatic nodes, muscle, oral mucosa, pancreas, prostate, small intestine, spleen, thymus, uterus/cervix.)
- Ionization Chamber
The interactions of charged particles (either direct charged particles or secondary Particles produced by interactions with photons or neutrons) with a gas lead to ionized and excited molecules along the path. The important information is the total number of Electron-ion pairs created along the track of the radiation. The W-value is defined as the average energy lost by the incident particle per ion pair formed. Due to the competing mechanism of the energy loss, i.e. excitation, W-value is always greater than the ionization energy. The table below shows W-values for common gases.
Ionization potential and W-values for different gases.
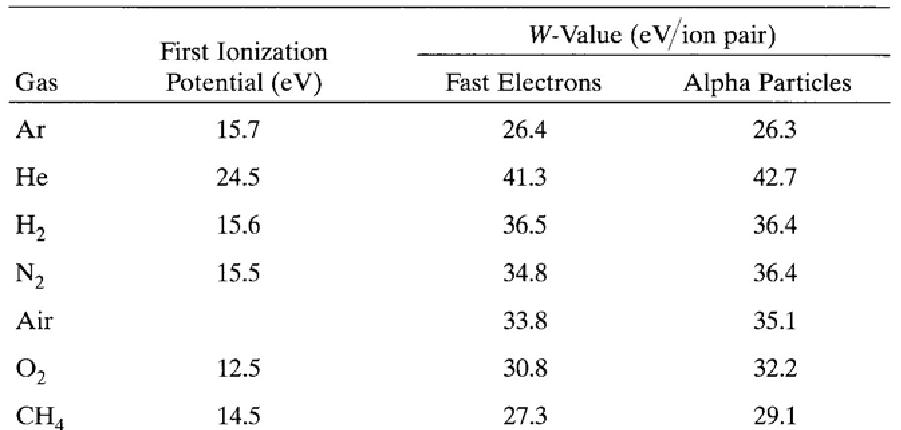
Charge transfer occurs when a positive ion encounters a neutral molecule. In this case, an electron is transferred from the neutral molecule to the ion. When a free electron meets a gas molecule that attaches electrons easily, a negative ion is formed. Oxygen is a good example. Thus, free electrons diffusing in air are rapidly converted to negative ions. In contrast, nitrogen, hydrogen and noble gases have low electron attachment coefficients, and make electrons continuously migrate. To collect electron-ion pairs produced in a gas-filled detector, an electric field must be applied. If the electric field inside the detector is strong enough that recombination becomes negligible, all the charges are efficiently collected without loss. The steady state current measured at this condition is called ionization current, which is the basic principle of the DC ion chamber.
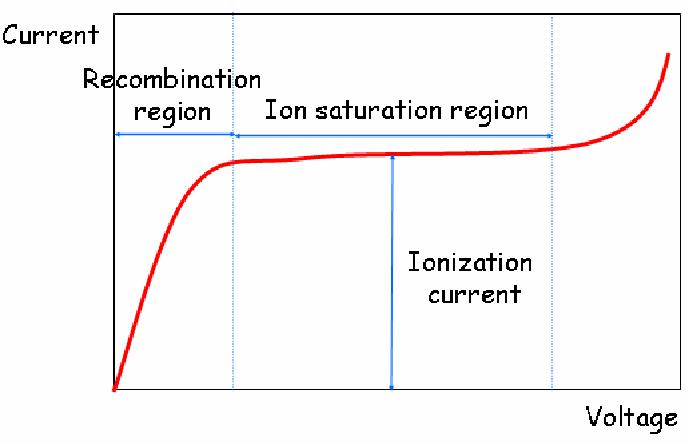
- Operation of DC ion chambers:
When an ion chamber is operated in direct current mode, the negative charges can be collected either as free electrons or as negative ions. Thus, any filling gas could be used. Air is the most common filling gas. Typical ionization currents in most applications are very small (1 pA or less). Thus, the leakage current through the insulator can perturb measuring the ionization current.
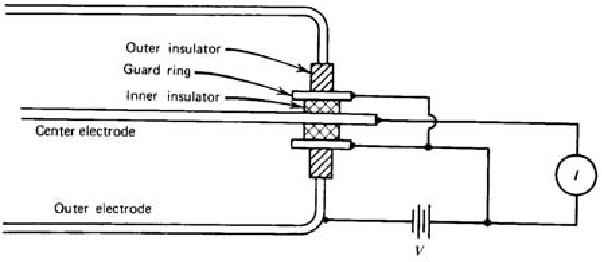
Fig. 3.3. Ion chamber employing guard ring
Ion chamber are used between 0.1cc to 1.0cc for routine usage.
For example, if we want to keep the leakage current at 1 % level of an ionization current of 1 pA for an applied voltage of 100 V, the insulator should have a resistance of 1016
ohms. Moreover, moisture or surface contaminants can make additional troubles. Practically, a guard ring is employed to reduce this insulator leakage in low current applications of ion chambers. As shown in Fig. 3.3, the insulator is segmented into two parts. Most of the applied voltage appears across the outer insulator, for which the resultant leakage current does not contribute to the measured current I. The magnitude of the ionization current is too small to be measured using standard
galvanometer techniques. An electrometer, i.e. highly sensitive electronic voltmeter, indirectly measures the current by sensing the voltage drop across a series resistance (109 ~ 1012 ohms) as shown in Fig. 3.4. Provided the ion current does not change for several values of the time constant RC, its steady state value is given by I = VR/R.
- Geiger-Muller Counter
Geiger-Muller (G-M) Counters produce a large voltage pulse because the numbers of ion pairs produced are greatly amplified, by the phenomenon known as gas multiplication. Gas multiplication is induced by a strong applied electric field.
After creation by radiation interaction, both electrons and ions make many collisions with neutral gas molecules until collected. When the electric field is strong enough, electrons
get high kinetic energies between collisions and eventually can ionize neutral molecules as illustrated in Fig. 3.8 while ions have low mobility and they attain very little energy
between collisions. The two electrons can then be accelerated and further ionizations can be caused. The process takes the form of a cascade (Townsend avalanche). The electric
field must be stronger than the threshold. The fractional increase in the number of electrons per unit path length is governed by the Townsend Equation:
dn/n = αdx
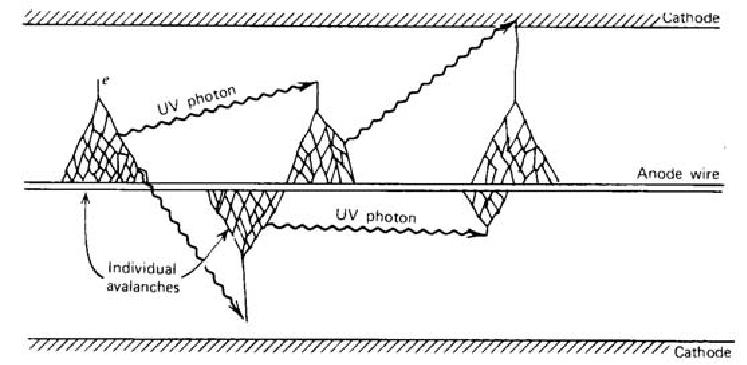
The excited gas molecules produced during a Townsend avalanche in addition to the secondary ions return to the ground state by emitting photons, which plays an important role in the Geiger discharge. The energy of photons is in the visible or ultraviolet region. The photon can be absorbed either by another gas molecule or by the cathode wall through photoelectric effect, which creates another free electron. This new electron is accelerated by the electric field inside the detector and triggers another avalanche. The chain reaction of the avalanche leads to the Geiger discharge. In a Geiger discharge, the rapid propagation of the chain reaction forms many avalanches throughout the multiplying region that surrounds the anode. Therefore, the discharge grows to envelope the entire anode wire.
The termination of a Geiger discharge is induced by the positive ions. The mobility of the positive ions is much less than that of the electrons, and they remain motionless during the free electron collection time. As the concentration of the positive ions increases near the anode, the space charge effect reduces the strength of the electric field and eventually terminates the discharge. Since the termination condition requires a fixed amount of positive charge, the discharge chain continues until it meets the termination condition regardless of the initial number of electron-ion pairs, which were created through interaction with the incident radiation. Therefore, all output pulses of a G-M counter have the same amplitude, which makes a G-M counter unsuitable for radiation spectroscopy.
When positive ions arrive at the cathode, they are neutralized by combining with electrons from the cathode surface, and an amount of energy equal to the ionization energy of the gas minus the work function of the cathode, the energy required to extract the electron from the cathode, is liberated. If this energy is bigger than the cathode work function, another free electron can emerge from the cathode and the free electron triggers another avalanche, leading to a second Geiger discharge.
To prevent this multiple pulsing problem, a quenching gas is added to the primary fill gas. The ionization energy of the quench gas is smaller and the molecular structure is more complex (usually, ethyl alcohol and ethyl formate). When positive ions of the primary gas collide with the neutral quench gas molecules, the positive charge is
transferred to the quench gas (in fact, an electron is transferred to the positive charge). Then all the ions arriving at the cathode will be those of the quench gas and after neutralization, the excess energy is preferentially used for disassociation of the complex molecules. The requirements for primary filling gases are same as those for proportional counters. The noble gases are widely used as the principal component.
- TLD/OSL Dosimeters
Thermo luminescent Dosimeter (TLD):
Some dosimeters contain storage phosphors, in which a fraction of the electrons raised to excited states by ionizing radiation, become trapped in excited states. When these trapped electrons are released, either by heating or by exposure to light, they fall to lower energy states with the emission of light. TLD’s having wider advantage than the fim badges. The most commonly used TLD material for personnel dosimetry is lithium fluoride (LiF). LiF TLDs have a wide dose response range of 10 ~Sv to 103 Sv (l mrem to 105 rem) and are reusable. Another advantage of LiF TLDs is that the effective atomic number is close to that of tissue; therefore, the dose to the dosimeter is close to the tissue dose over a wide energy range. TLDs do not provide a permanent record, because heating the chip to read the exposure removes the deposited energy.
TLD’s are routinely used in nuclear medicine as extremity dosimeters; a finger ring containing a chip of LiF is worn on the hand expected to receive the highest exposure during radio-pharmaceutical preparation and administration. TLDs are also the dosimeter of choice when longer dose assessment intervals (e.g., quarterly) are required.
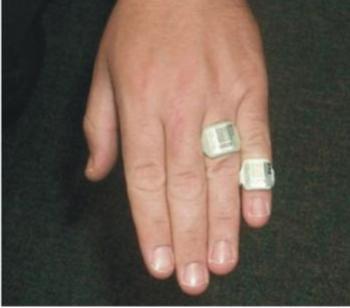
Figure shows the TLD ring used in finger to monitor.
A TLD badge based on CaSO4: Dy Teflon discs has been designed and is in regular Personnel Monitoring use since 1975. The TLD badge has shown satisfactory performance for monitoring beta and gamma doses of radiation workers. At present about 40,000 radiation workers are covered with TLD monitoring service in our nuclear industry, medical and industrial institutions as well as research institutions. The complete Personnel Monitoring TLD badge consists of a TLD card and a plastic cassette for holding the TLD card. The badge is affixed to the clothing of a person with the help of a crocodile clip attached to the badge. Figure below shows a picture of TLD badge used in India.

- Optical stimulated luminescence (OSL):
Dosimeters using optically stimulated luminance (OSL) have recently become commercially available as an alternative to TLDs. The principle of OSL is similar to that of TLD’s, except that the light emission is stimulated by laser light instead of by heat. Crystalline aluminum oxide activated with carbon (Alz03:C) is commonly used. Like TLDs, OSL dosimeters have a broad dose response capability, and are capable of detecting doses as low as 10 ~Sv (1 mrem). However, OSL dosimeters have certain advantages over TLDs in that they can be reread several times and an image of the filter pattern can be produced to differentiate between static (i.e., instantaneous) and dynamic (i.e., normal) exposure.
REFERENCES
1. The National Academies Press. (2008). Basic Concepts in Radiation Physics, Biology and Epidemiology, Chapter 3, pg.43. Retrieved on (2018-15-08).
Home > Radiation
Protection and Quality Assurance > Radiation Physics and Biology
FREE Infographic What successful people believe. What successful people do
Dictionary of Cancer Terms
Need help understanding a word? Here is an electronic resource that gives meaning to Cancer terms and their usage.

StrengthsFinder 2.0
